2025-05-26
Nature Biomedical Engineering: Tension- induced Directional Migration of Hepatic Stellate Cells Potentially Coordinate Liver Fibrosis Progression
杜亚楠课题组构建具有肝小叶结构的肝纤维化体外模型并探究张力介导的细胞定向迁移促进肝纤维化机制
2025年5月23日,清华大学生物医学工程学院教授、清华-北大生命联合中心研究员杜亚楠团队在 Nature Biomedical Engineering (《自然生物医学工程》) 期刊发表题为Tension- induced Directional Migration of Hepatic Stellate Cells Potentially Coordinate Liver Fibrosis Progression的研究论文。
1. Background
Liver fibrosis represents a global health challenge. It is characterized by the excessive accumulation of extracellular matrix components, leading to the formation of fibrotic scar tissue that disrupts the normal physiological architecture of the liver. During the course of chronic liver injury, fibrosis gradually progresses to cirrhosis, which may further lead to liver failure or the development of hepatocellular carcinoma. Notably, the majority of liver fibrosis-related mortality occurs at advanced stages. Therefore, elucidating the mechanisms by which early-stage fibrosis progresses to cirrhosis is of significant clinical importance, as it may inspire novel therapeutic strategies and interventions.
Clinically, various staging systems for liver fibrosis are closely associated with the patterns of collagen deposition within the hepatic lobule. Among them, the METAVIR system and three other widely used fibrosis scoring systems (Figure 1) indicate that, in the early stages, collagen deposition predominantly occurs in a punctate manner around the portal tracts. In contrast, in advanced stages, collagen distribution exhibits a directional “portal-to-portal” pattern, resulting in the formation of fibrous septa. However, the mechanisms underlying this directional collagen distribution remain unclear.
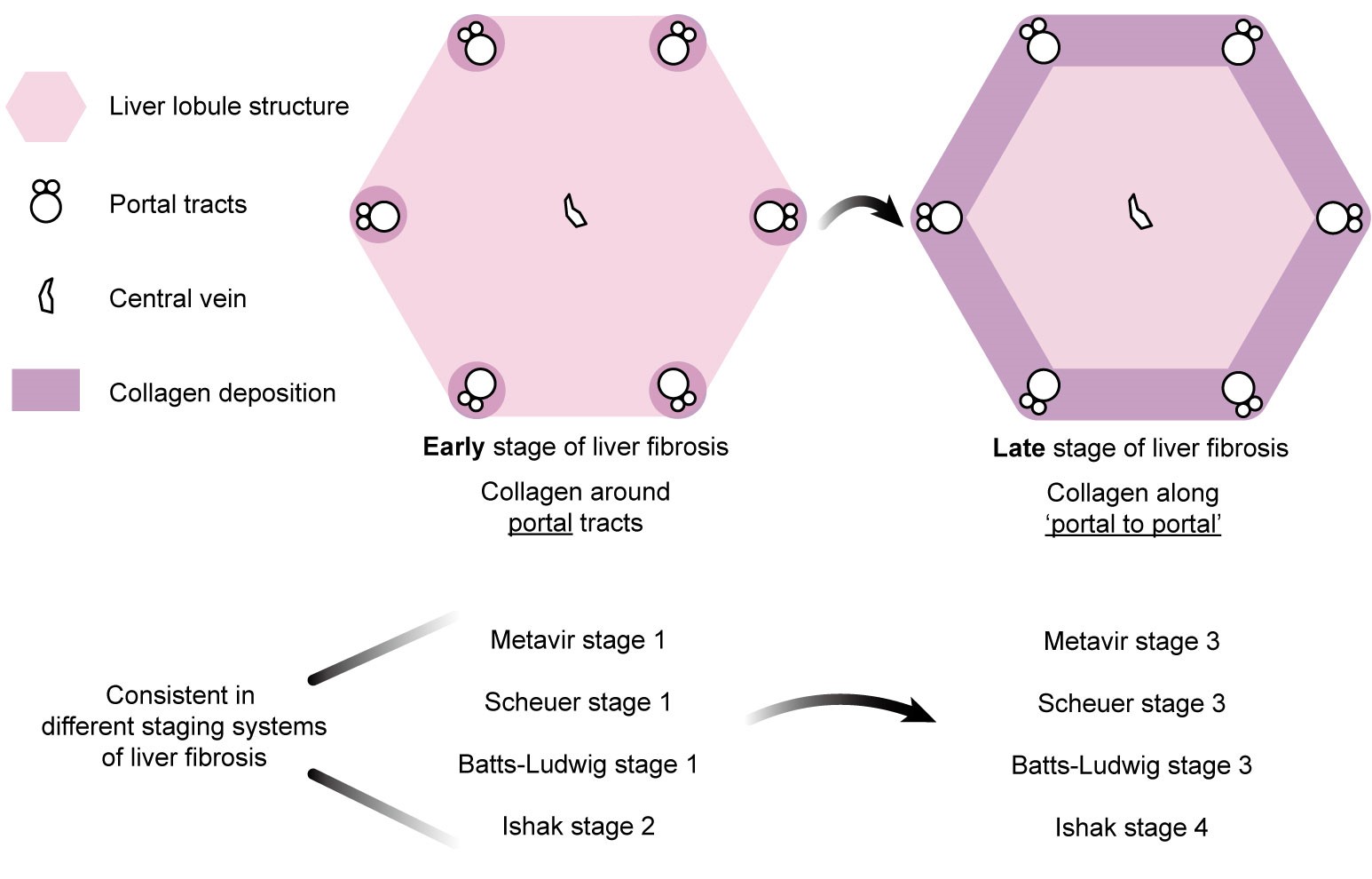
Figure 1. The spatial distribution of collagen in early and late stages of liver fibrosis exhibits directionality across commonly used clinical staging systems.
2. Research Aim
To develop an in vitro model with hepatic lobule architecture. Most existing in vitro liver fibrosis models lack the structural features of hepatic lobules and are therefore unable to recapitulate the directional distribution of collagen and the formation of fibrous septa observed during the progression of fibrosis.
To investigate the regulatory role of biomechanical factors in the directional collagen deposition during liver fibrosis. Current mechanistic studies on liver fibrosis progression primarily focus on the diffusion of biochemical factors. However, the isotropic diffusion of such factors and the resulting activation of hepatic stellate cells fail to account for the “portal-to-portal” directional pattern of collagen deposition. In contrast, cell-mediated tension sensing of the surrounding extracellular matrix, which can be anisotropic, may provide a mechanistic explanation for this directional collagen alignment.
3. Overview
This study achieved a multiscale systematic investigation (Figure 2).
Organ scale: In a mouse model of liver fibrosis, we characterized the directional “portal-to-portal” collagen deposition and the spatial distribution of hepatic stellate cells.
Tissue scale: For the first time, we established an in vitro liver fibrosis model with hepatic lobule architecture, capable of recapitulating the in vivo directional distribution of hepatic stellate cells and collagen, as well as the formation of fibrous septa.
Cellular scale: Using an in vitro collagen stretching system to mimic the strain generated by remodeled collagen in fibrotic liver tissue, we found that hepatic stellate cells exhibited directional migration along the remodeled collagen. Both their migration speed and activation status were correlated with the magnitude of strain.
Molecular scale: We demonstrated that liquid–liquid phase separation of the mechanosensitive protein LIMD1 is involved in regulating the directional migration of hepatic stellate cells.
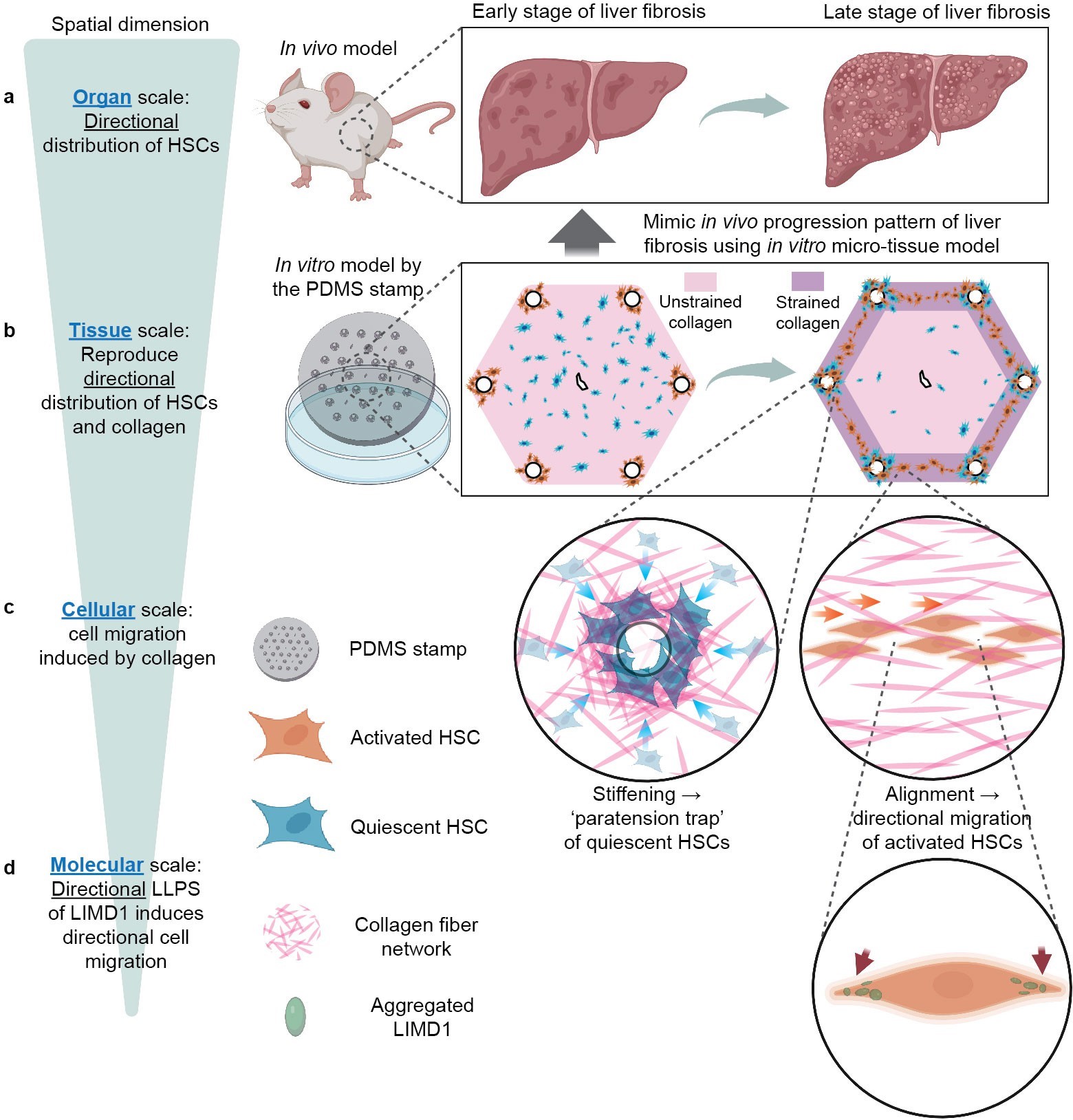
Figure 2. Schematic figure.
4. Results
In this study, a carbon tetrachloride (CCl₄)-induced mouse model of liver fibrosis was used to characterize the pathological features at both early and late stages. Hepatic stellate cells (HSCs), the principal effector cells in the progression of liver fibrosis, secrete large amounts of collagen that contribute to its deposition. Our findings reveal that, during the early stage of fibrosis, quiescent HSCs are diffusely distributed throughout the hepatic lobules, while activated HSCs are predominantly localized near the portal tracts. In the late stage, a portion of quiescent HSCs remains near the portal regions, whereas activated HSCs, along with remodeled collagen, exhibit a directional “portal-to-portal” distribution pattern, forming fibrous septa. To recapitulate this phenomenon, we developed a biomimetic “hepatic lobule” in vitro model using microtissue engineering techniques. This model successfully reproduces the spatial distribution patterns and dynamic changes of both collagen and hepatic stellate cells observed in vivo (Figure 3).

Figure 3. The in vitro model recapitulates the “hepatic lobule” architecture and simulates the directional distribution and dynamic changes of hepatic stellate cells and collagen during early and late stages of liver fibrosis.
To mimic the hexagonal structure of hepatic lobules, a micro-scale PDMS (polydimethylsiloxane) stamp was designed and fabricated in this study. The fabrication process integrated two techniques: laser engraving, which provides high processing depth but low resolution, and photolithography, which offers high resolution but limited processing depth (Figure 4).
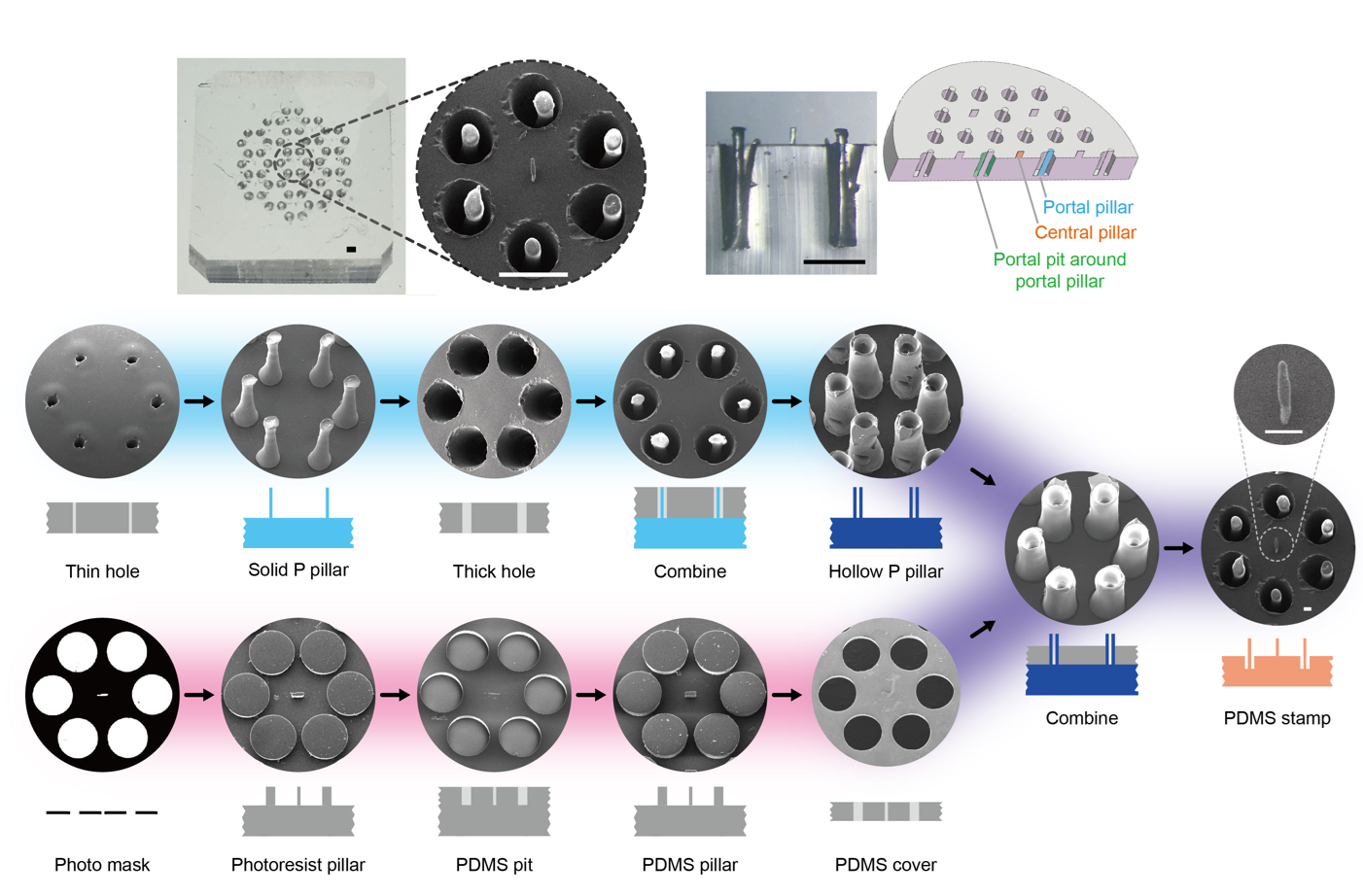
Figure 4. PDMS stamp used for the fabrication of the in vitro biomimetic “hepatic lobule” model.
The PDMS stamp functions similarly to stamping ink on paper. After cells settle into the stamp’s perforated structures, they can be transferred onto a collagen substrate, thereby forming an in vitro model that mimics early-stage liver fibrosis. In this model, activated hepatic stellate cells are concentrated around the portal regions, while quiescent stellate cells are diffusely distributed throughout the hepatic lobule (Figure 5).
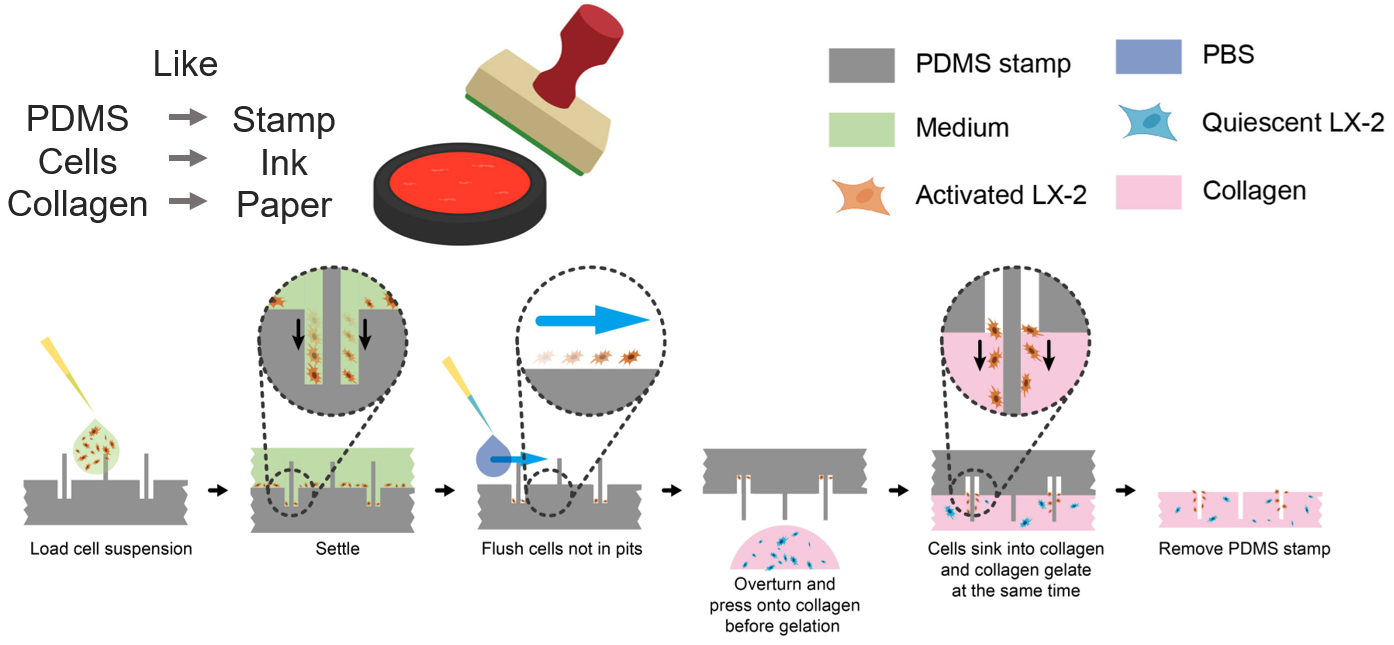
Figure 5. Reconstitution of early-stage hepatic stellate cell distribution in liver fibrosis using the PDMS stamp.
Given the critical regulatory role of macrophages in the progression of liver fibrosis, this study also incorporated M1-polarized macrophages into the microtissue model for co-culture. The results showed that these macrophages exerted a certain inhibitory effect on the progression of liver fibrosis (Figure 6).

Figure 6. Co-culture of M1-type macrophages with the liver fibrosis microtissue model.
Subsequently, the study investigated whether biomechanical factors are involved in regulating cell distribution within the in vitro liver fibrosis model (Figure 7). Using laser microdissection, it was observed that collagen fibers did not separate when incised parallel to the “portal-to-portal” axis, whereas a noticeable opening occurred when cut perpendicular to this direction. This suggests the presence of tensile stress along the “portal-to-portal” direction, which precedes and potentially guides the formation of the strip-like, directional distribution of cells.
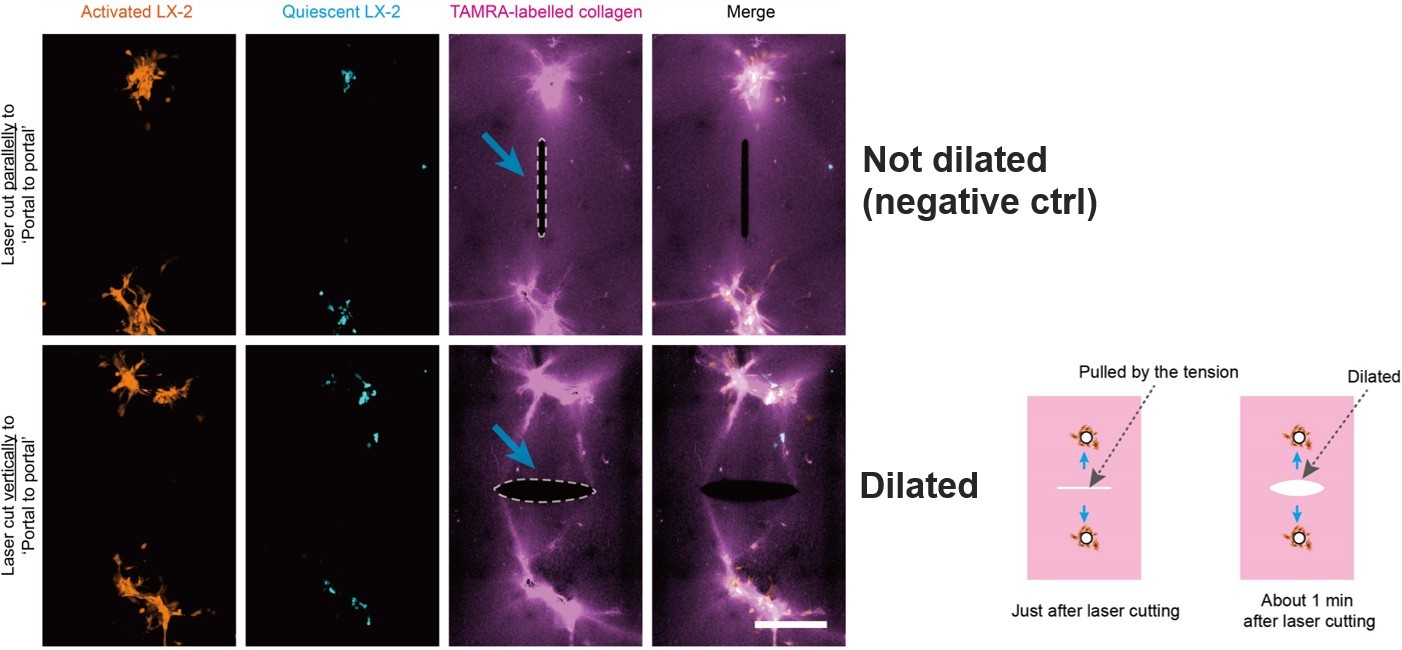
Figure 7. Laser microdissection reveals the presence of tensile stress along the “portal-to-portal” axis.
During subsequent culture after laser cutting, it was observed that both the uncut group and the group cut parallel to the “portal-to-portal” direction maintained the characteristic cord-like distribution of cells along this axis. However, in the group with cuts perpendicular to the “portal-to-portal” axis, cells redistributed along a new “portal-to-incision edge” direction. This pattern aligns with the tensile stress distribution predicted by finite element analysis (Figure 8). These findings further suggest that tension is a key factor driving the directional “portal-to-portal” distribution of activated hepatic stellate cells in the in vitro liver fibrosis model.
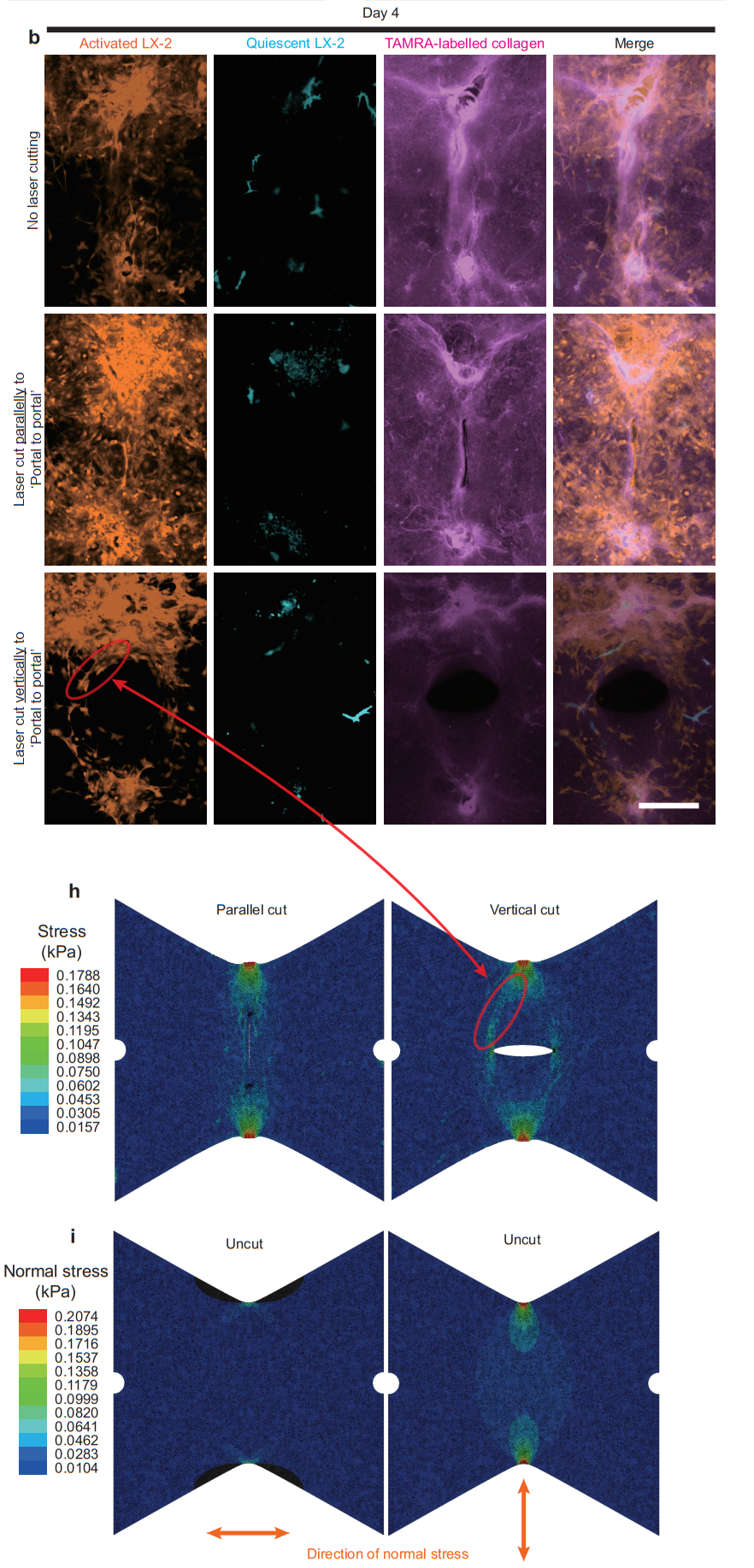
Figure 8. Laser microdissection suggests that the distribution of tensile stress regulates cell distribution.
Subsequently, to precisely investigate the interactions among tension, collagen, and cells, a stretching device compatible with confocal imaging was designed. This system was used to systematically study characteristic changes in collagen, such as stress-induced stiffening and directional alignment, under biomimetic strain (Figure 9).
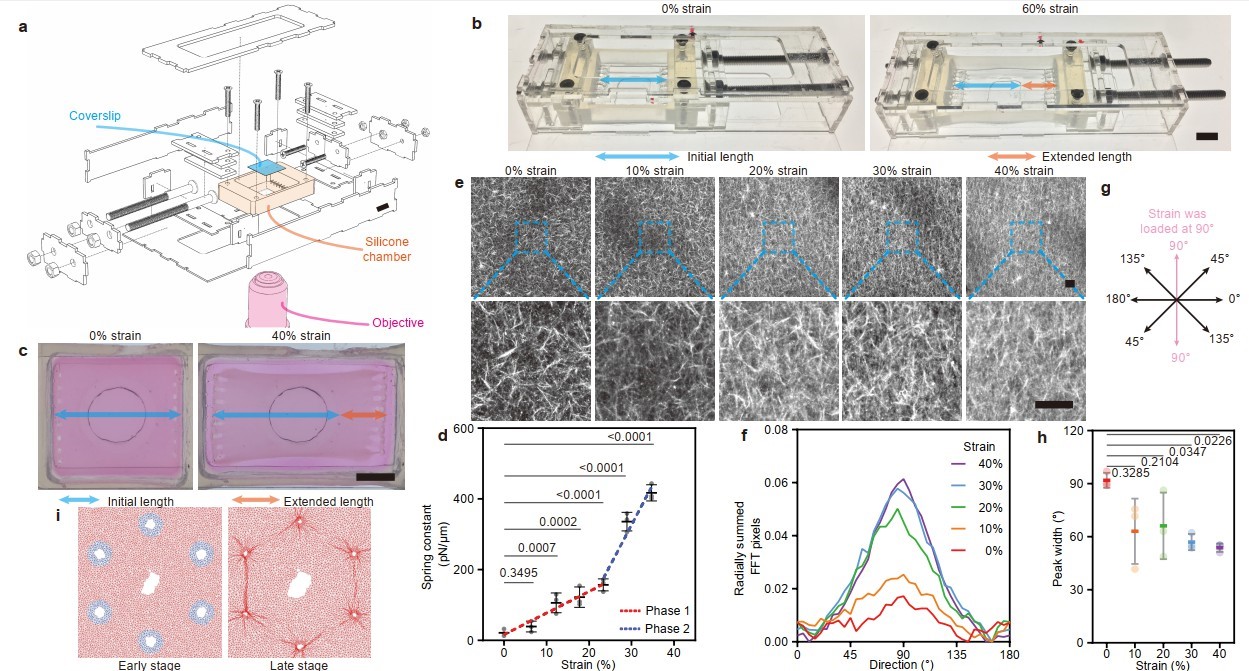
Figure 9. Tensile stress induces stress stiffening and directional alignment of collagen.
After miniaturizing the stretching device to be compatible with live-cell imaging platforms, the migration behavior of hepatic stellate cells on stretched, remodeled collagen was systematically characterized. For quiescent hepatic stellate cells, migration speed initially increased and then decreased with increasing strain, whereas the migration speed of activated hepatic stellate cells was not affected by strain. Additionally, both quiescent and activated cells exhibited migration directionality biased toward the stretch axis as the stretching intensity increased (Figure 10). These findings potentially explain how tensile stress regulates collagen remodeling and the dynamic spatial distribution of quiescent and activated hepatic stellate cells.
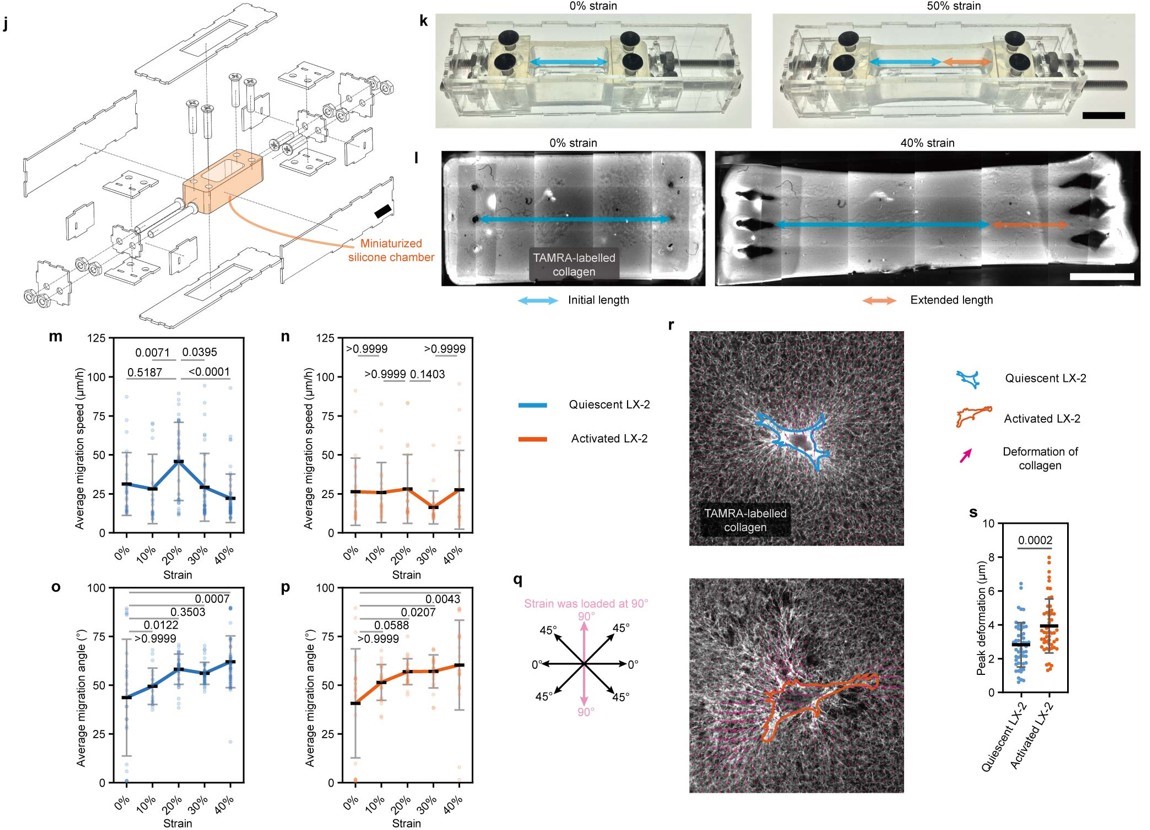
Figure 10. Characterization of hepatic stellate cell migration on collagen under different strains using a miniaturized stretching device.
Finally, the molecular mechanisms potentially involved in directional cell migration on collagen were investigated. Physical directional cues are transmitted into the cell via integrins that bind to collagen, activating the liquid-liquid phase separation (LLPS) of the force-responsive protein LIMD1 associated with integrins. This leads to the localized clustering of LIMD1, which recruits additional focal adhesion components and binds to actin filaments, thereby promoting directional migration of cells along the axis of tension. This mechanism was further validated by immunofluorescence staining, fluorescence recovery after photobleaching (FRAP) experiments, and gene knockout studies (Figure 11).
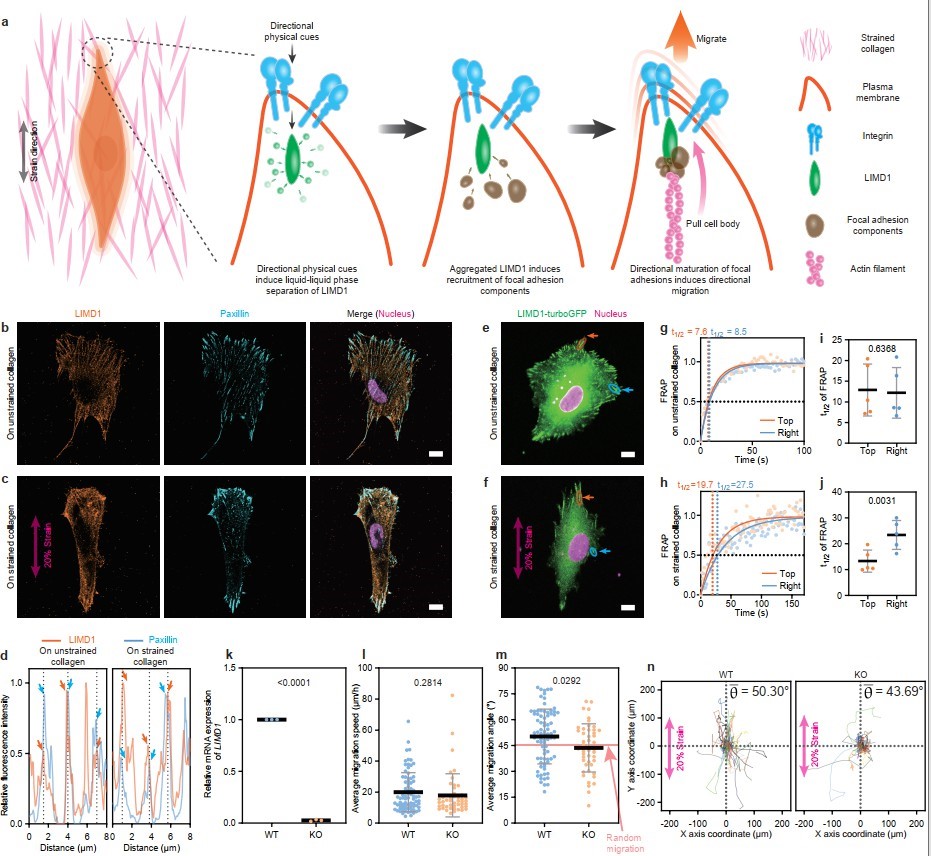
Figure 11. Molecular mechanism underlying directional migration of cells on stretched collagen.
In summary, this study developed an in vitro liver fibrosis model with lobular architecture and proposed and validated a potential mechanism explaining the directional distribution of cells and collagen in both early and late stages of liver fibrosis. Activated hepatic stellate cells localized in the portal tracts generate tensile forces that induce collagen stress stiffening and alignment. The stress stiffening effect promotes the migration and retention of quiescent hepatic stellate cells, which are diffusely distributed in the lobule, toward the portal area, resulting in their partial aggregation in the late stage. Meanwhile, the collagen alignment effect directs activated hepatic stellate cells to migrate along the tension axis, leading to a “portal-to-portal” directional distribution and the formation of fibrous septa (Figure 12). This study provides valuable insights into the mechanisms driving liver fibrosis progression and the development of potential novel therapeutic interventions.
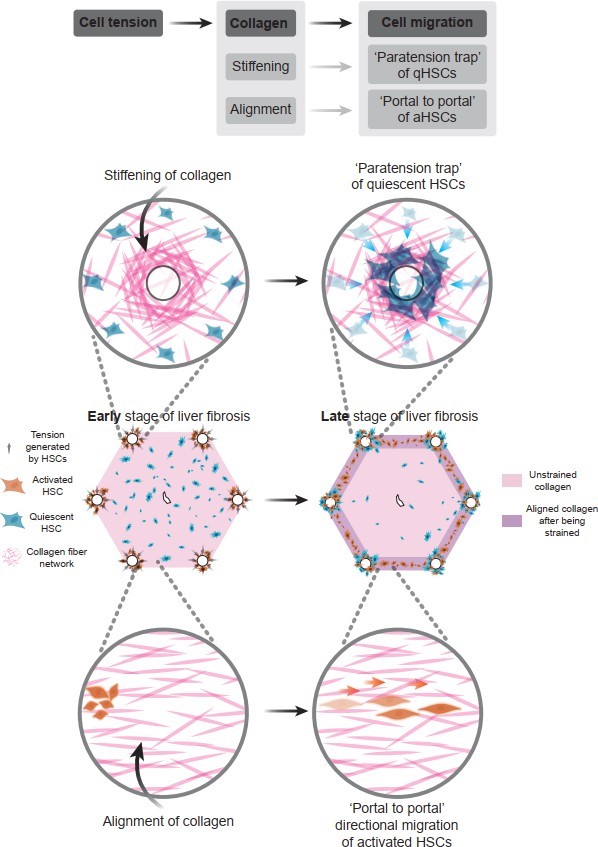
Figure 12. Summary towards underlying mechanism of how cellular tension regulates collagen remodeling and cellular distribution during liver fibrosis progression
Professor Yanan Du from the School of Biomedical Engineering at Tsinghua University and researcher at the Tsinghua-Peking University Joint Center for Life Sciences is the corresponding author of this article. Dr. Lyu Zhou from the School of Life Sciences at Tsinghua University is the first author. Important contributions to this study were made by doctoral student Ziao Shi, undergraduate Xuesi Yang from the School of Aerospace, doctoral students Jia’nan Zeng and Zhifeng You from the School of Biomedical Engineering, doctoral students Yuying Zhang, Zhiqiang Liu, and Yudi Niu from the School of Medicine, master’s student Hongsheng Yu from the School of Medicine, and doctoral student Jinliang He from the School of Life Sciences, as well as Zhiyue Zhu from the University of Toronto. Professor Congying Wu from the Institute of Systems Biomedicine at Peking University’s School of Basic Medical Sciences provided important guidance for this research. This study was supported by the National Natural Science Foundation of China through the Distinguished Young Scholars Program, Key Program, and International Cooperation Program (grant numbers 82125018, 32430058, and 82061148010).
DOI: https://doi.org/10.1038/s41551-023-01019-z
Relevant reference publications previously published by Prof. Yanan Du’s laboratory on in vitro pathological models of liver fibrosis:
[1] Liu, L., You, Z., Yu, H., Zhou, L., Zhao, H., Yan, X., ... & Du, Y. (2017). Mechanotransduction-modulated fibrotic microniches reveal the contribution of angiogenesis in liver fibrosis. Nature materials, 16(12), 1252- 1261.
[2] Liu, L., Yu, H., Zhao, H., Wu, Z., Long, Y., Zhang, J., ... & Du, Y. (2020). Matrix-transmitted paratensile signaling enables myofibroblast–fibroblast cross talk in fibrosis expansion. Proceedings of the National Academy of Sciences, 117(20), 10832-10838.
[3] Long, Y., Niu, Y., Liang, K., & Du, Y. (2022). Mechanical communication in fibrosis progression. Trends in cell biology, 32(1), 70-90.
[4] Lyu, C., Kong, W., Liu, Z., Wang, S., Zhao, P., Liang, K., ... & Du, Y. (2023). Advanced glycation end- products as mediators of the aberrant crosslinking of extracellular matrix in scarred liver tissue. Nature biomedical engineering, 7(11), 1437-1454.

