2023-04-11
Nature Biomedical Engineering
Identification and Reconstruction of AGE-crosslinked extracellular matrix revealing the aberrant mechano-microenvironment in cirrhotic liver
Liver cirrhosis, which is developed from liver fibrosis, has become the 13th deathly disease in the world and the 10th deathly disease in China. Late-stage cirrhosis is prone to transform into liver cancer, and the number of deaths of liver cancer patients is around 400,000 in China every year, accounting for about half of the deaths of liver cancer patients worldwide. Currently, there is no effective clinical treatment for liver cirrhosis except for whole-liver transplantation. The formation of highly crosslinked extracellular matrix (ECM) in the cirrhotic liver tissue is one of the critical reasons hindering the recovery of cirrhosis. A number of studies have been trying to treat liver cirrhosis by inhibiting the Lysyl Oxidase (LOX)-mediated crosslinking reactions, and therefore inhibiting the formation of crosslinked collagen scar tissues. But none of them has achieved satisfactory therapeutic effect in clinical trials. This indicates that the highly crosslinked extracellular matrix of cirrhosis is still poorly understood. The liver is an essential organ responsible for glucose metabolism, and cirrhosis causes disorders of glucose metabolism in liver tissues. About 30% of cirrhotic patients have concomitant diabetes, suggesting potential associations between Advanced Glycation End-products (AGE)-crosslinking reactions driven by reducing sugars (e.g., glucose) and the formation of highly crosslinked collagen scars in cirrhotic liver tissues. In-depth investigation of the role of crosslinking reactions on collagen scar formation in cirrhotic liver tissues is promising to reveal the mechanism of liver fibrosis progression and provide new potential targets for clinical treatment.
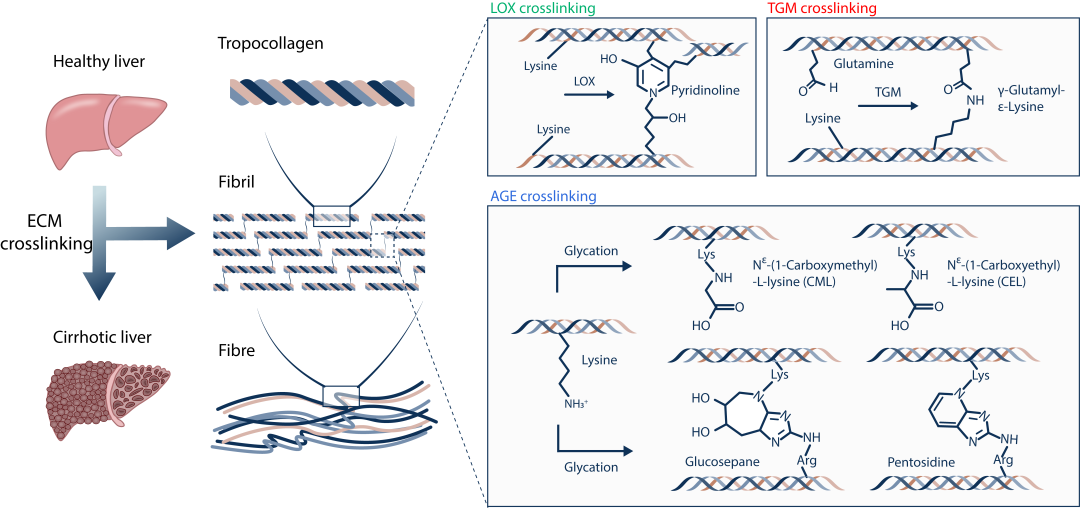
Figure 1. Numerous crosslinking reactions are involved in the formation of highly crosslinked collagen scar in cirrhotic liver tissue during fibrosis progression. The crosslinking reactions mediate the formation of crosslinks linking collagen fibrils. The crosslinked collagen matrix usually shows increased mechanical strength and resistance to proteinase-mediated degradation. Green color, Pyridinoline crosslinks generated by LOX crosslinking. Red color, γ-Glutamyl-ε-Lysine crosslinks generated by TGM crosslinking. Blue color, CML,CEL,GLucosepane,Pentosidine crosslinks generated by AGE crosslinking.
Our group has identified Advanced glycation end-products (AGE) as mediators of the aberrant crosslinking of extracellular matrix in scarred liver tissue. The study revealed for the first time that "highly AGE-crosslinked collagen matrix is an important pathological feature of liver cirrhosis". By characterizing crosslinked liver ECM in vivo and reconstructing the AGE crosslinked collagen matrix model in vitro, it was revealed that AGE crosslinking could significantly alter the structural and mechanical properties of collagen fibrils, leading to an abnormal biophysical microenvironment that regulates cellular phenotypes and functions. It was also shown that inhibition of AGE crosslinking reaction could potentially inhibit the formation of crosslinked collagen scar and alleviate the progression of liver fibrosis.
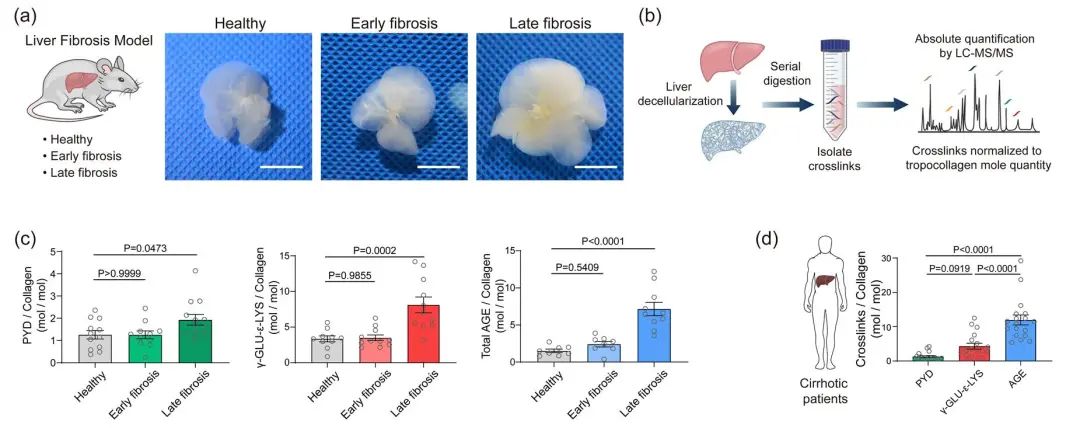
Figure 2. Quantification of crosslinking degree of liver ECM. (a) Decellularization of mouse liver ECM; (b) Quantification of crosslinking degree of liver ECM by using AQMC method. (c) Quantitative results of the crosslinking degrees of liver ECM in a liver-fibrotic mouse model. (d) Quantitative results of the crosslinking degrees of liver ECM in clinical samples from liver-cirrhotic patients. Results indicate the high degree of AGE crosslinking of cirrhotic liver ECM.
In this study, we first investigated various crosslinking reactions that may be involved in the formation of highly crosslinked scar tissue in cirrhotic tissues 1.2, such as the crosslinking reactions mediated by lysyl oxidase (LOX), transglutaminase (TGM), and advanced glycation end-products (AGE) (Figure 1). In order to precisely characterize the crosslinking degree of the Extracellular Matrix (ECM) and avoid the unexpected interference caused by cellular components and metabolites in liver tissue during the quantification process, we effectively isolated the liver ECM from healthy and cirrhotic liver tissues by using the optimized tissue decellularization technique. By combining the liquid chromatography-mass spectrometry (LC-MS), we developed an absolute quantification of Matrix-specific Crosslinking (AQMC) method to precisely quantify the crosslinking degree of liver ECM. By using the AQMC method, we, for the first time, demonstrate that the highly AGE-crosslinked liver extracellular matrix is a critical pathological characteristic of liver cirrhosis, and this conclusion was validated in clinical samples from cirrhotic patients and two different animal models of liver fibrosis (Figure 2). Quantitative analysis showed that AGE crosslinking could play a more significant role than LOX crosslinking and TGM crosslinking reactions in the formation of collagen scar in cirrhotic liver tissues.
In order to reveal the regulatory effects of AGE crosslinking on the extracellular matrix, we reconstructed AGE-crosslinked collagen matrices with different crosslinking degrees in vitro and reproduced the pathological characteristics of the extracellular matrix during the development of liver fibrosis in vivo in terms of viscoelasticity, structural properties and crosslinking degrees. By using cryo-electron microscopy and optical tweezer techniques, we characterized the regulatory effects of AGE crosslinking on collagen matrix from the bulk level to the single-fibril level, revealing that AGE crosslinking lead to the intertwining of adjacent fibers, and the formation of thick fibril bundles, resulting in a reduced stress relaxation rate of crosslinked collagen fibrils, which makes AGE crosslinked collagen fibrils resist to cell-mediated remodeling (Figure 3).
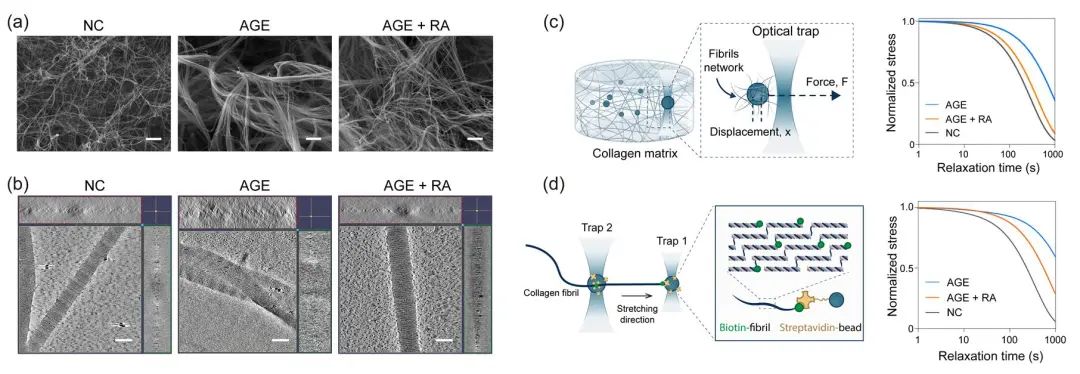
Figure 3. The effects of AGE crosslinking on biomechanics of collagen matrix. (a) SEM images of the reconstructed collagen matrix in vitro. (b) 3D reconstructed models of collagen fibrils generated by Cryo-EM images. (c) Characterization of the viscoelasticity of the bulk collagen matrix by using single-trap optical tweezers. (d) Characterization of the viscoelasticity of the single collagen fibrils by using double-trap optical tweezers.
The previous work by our team suggested that ‘para-tensile’ biomechanical signals mediated by extracellular matrix in liver tissue could modulate cellular behavior and further exacerbate fibrosis progression 3,4. This suggests that AGE crosslinking may be involved in this biomechanical regulation by modulating the properties of the crosslinked collagen matrix. Macrophages are important regulators of liver fibrotic disease, whose phenotypes and functions are sensitively regulated by the mechano-signals mediated by extracellular matrix 5. In this study, we particularly investigated the regulatory effects of AGE crosslinked collagen matrix on macrophages. In vitro results showed that macrophages grown on AGE-crosslinked collagen matrix showed decreased extent to remodel and recruit the surrounding collagen fibrils, resulting in reduced cell-matrix adhesion molecules, reduced cytoskeleton assembly, and reduced expression of Histone Deacetylase 3 (HDAC3). This mechanotransduction leads to a downregulated type I immune response and an upregulated type II immune response in macrophages, which potentially exacerbates the fibrosis process. By testing potential compound candidates using our in vitro models, we found that the small molecule compound Rosmarinic Acid (RA) could inhibit the formation of AGE-crosslinked collagen matrix in vitro and in a liver-fibrotic mouse model in vivo. And an alleviation of liver fibrosis was also found to be accompanied by the inhibition of AGE-crosslinking mediated by RA treatment in the liver-fibrotic mouse model (Figure 4).
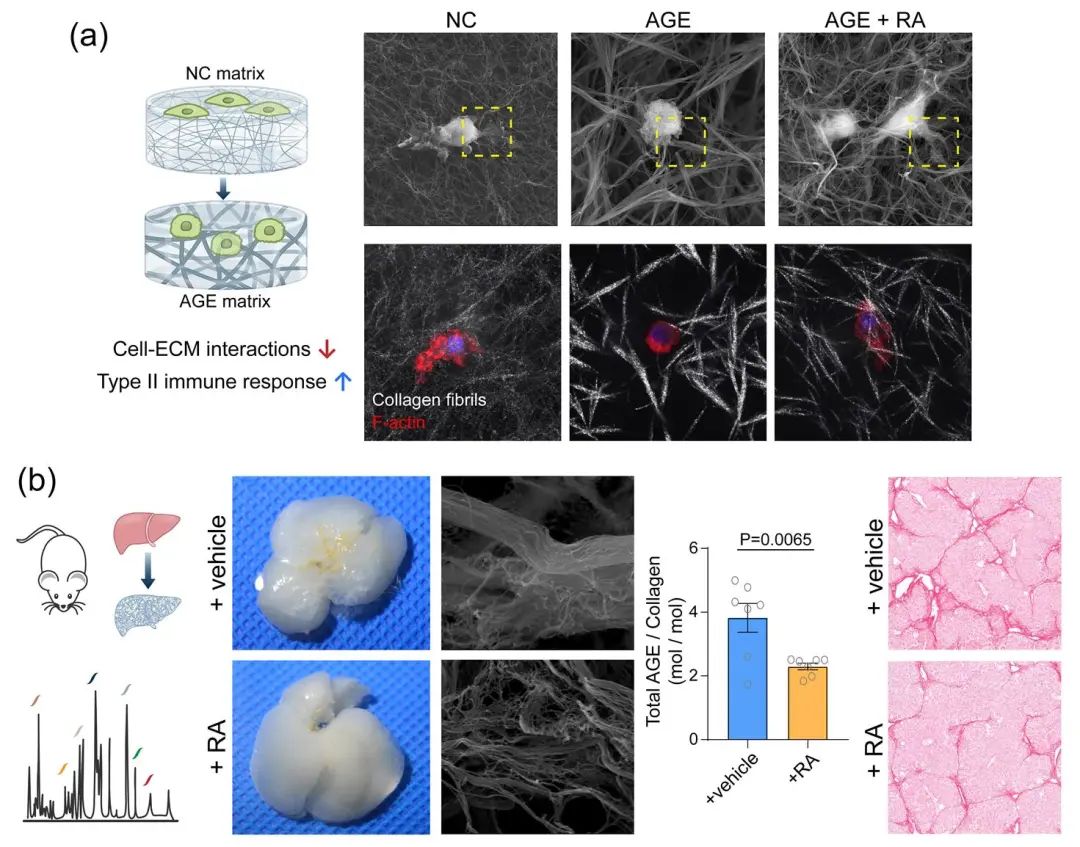
Figure 4. (a) AGE crosslinking could regulate the biomechanical properties of collagen matrix and the downstream immune response of macrophages. (b) Rosmarinic acid could inhibit the formation of AGE-crosslinked collagen scar and alleviate liver fibrosis in a mouse model.
In summary, considering there is a lack of effective clinical therapies for liver cirrhosis and being inspired by the clinical observation that cirrhosis is often accompanied by abnormal glucose metabolism, our research group developed a method to precisely quantify the crosslinking degree of the extracellular matrix of cirrhotic liver tissues, and we for the first time identified the highly AGE-crosslinked collagen matrix as an important pathological feature of liver cirrhosis. By reconstructing the in-vivo-mimicking AGE-crosslinked collagen matrix models, we revealed the regulatory effects of AGE crosslinking on the biomechanics of collagen matrix and its downstream modulatory effects on macrophages and fibrotic disease (Figure 5). Our results provide powerful tools for the characterization and mechanistic study of fibrotic disease, and provide new insights and potential targets for liver fibrosis diagnosis and treatment.
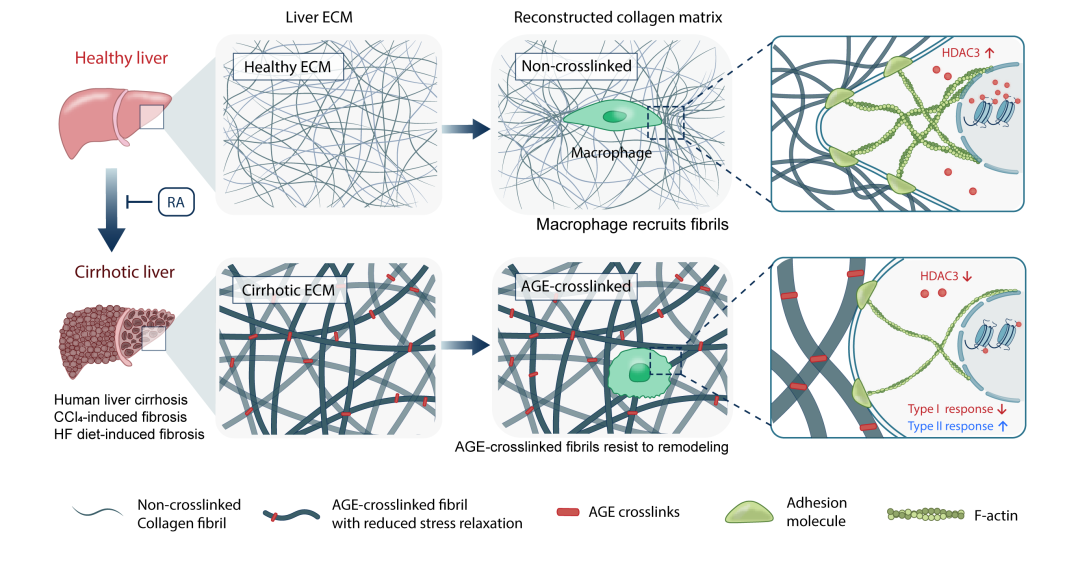
Figure 5. Graphical summary of this work.
DOI:https://doi.org/10.1038/s41551-023-01019-z
NBME News DOI:https://doi.org/10.1038/s41551-023-01119-w
References:
[1] Lyu C, Kong W, Liu Z, Wang S, Zhao P, Liang K, Niu Y, Yang W, Xiang C, Hu X, Li X, Du Y. Advanced glycation end-products as mediators of the aberrant crosslinking of extracellular matrix in scarred liver tissue. Nature Biomedical Engineering. 2023. DOI: 10.1038/s41551-023-01019-z (https://www.nature.com/articles/s41551-023-01019-z)
[2] Kong W, Lyu C, Liao H, Du Y. Collagen crosslinking: effect on structure, mechanics and fibrosis progression. Biomedical Materials. 2021, 16(6).
[3] Liu L, You Z, Yu H, Zhou L, Zhao H, Yan X, Li D, Wang B, Zhu L, Xu Y, Xia T, Shi Y, Huang C, Hou W, Du Y. Mechanotransduction-modulated fibrotic microniches reveal the contribution of angiogenesis in liver fibrosis. Nature Materials. 2017, 16(12): 1252-1261.
[4] Liu L, Yu H, Zhao H, Wu Z, Long Y, Zhang J, Yan X, You Z, Zhou L, Xia T, Shi Y, Xiao B, Wang Y, Huang C, Du Y. Matrix-transmitted paratensile signaling enables myofibroblast-fibroblast cross talk in fibrosis expansion. PNAS. 2020, 117(20): 10832-10838.
[5] Jiang S, Lyu C, Zhao, P., Li W, Kong W, Huang C, Genin GM, Du Y. Cryoprotectant enables structural control of porous scaffolds for exploration of cellular mechano-responsiveness in 3D. Nature Communications. 2019, 10(1): 3491.

